Overview
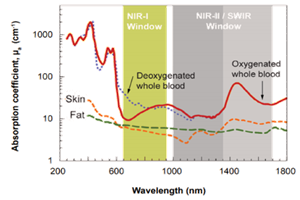
Working in the shortwave-infrared (SWIR) region of the spectrum affords researchers several advantages, including the abilities to circumvent unwanted fluorescence backgrounds and to probe more deeply into sample surfaces. The advent of deep-cooled camera systems that employ indium gallium arsenide (InGaAs) focal plane arrays (FPAs) has further increased the utility of various SWIR imaging and spectroscopy techniques for low-light-level scientific and industrial applications [1].
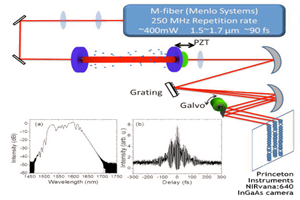
Recently, researchers at the Massachusetts Institute of Technology (MIT) published results of a study in which they leveraged the most advanced deep-cooled InGaAs FPA camera technology commercially available (see Figure 1) to evaluate novel InAs-based SWIR quantum dots. Working under the auspices of principal investigator Dr. Moungi G. Bawendi, lead authors Dr. Oliver T. Bruns and Dr. Thomas S. Bischof demonstrated that these new SWIR-emitting core/shell quantum dots (QDs) hold great promise for the next generation of in vivo SWIR imaging [2].

Dr. Bawendi’s laboratory, which is in the university’s Department of Chemistry, focuses on the fundamental science and leading-edge application of nanocrystals, especially semiconductor nanocrystals (i.e., quantum dots). The lab collaborates with a number of biology and medical groups to design nanocrystal probes that meet specific challenges, including utilization as molecular imaging agents in vivo [3].
This application note will summarize the lab’s use of a Princeton Instruments NIRvana scientific camera to perform the aforementioned evaluation of novel InAs-based SWIR emitting quantum dots as in vivo imaging agents.
Macroscopic & Microscopic SWIR Imaging
For macroscopic imaging of the vasculature of the subject’s (i.e., the mouse’s) brain through skin and skull, the MIT researchers built a custom experimental setup to direct the light emitted through various filters to a NIRvana camera equipped with various C-mount lenses. To eliminate excess light whilst enabling manipulation of the field-of-view during operation, the entire macroscopic imaging assembly was surrounded by a partial enclosure.
Microscopic imaging was performed using the NIRvana camera attached to the sideport of an inverted microscope, and either a 10x or a 2x objective. A fiber-coupled 808 nm laser diode was utilized for illumination, with a speckle remover employed. A dichroic filter directed the excitation light to the sample. Then a 1000 nm long-pass filter selected the emission light
The camera’s InGaAs array was cooled to -80°C for microscopic imaging, the analog-to-digital conversion rate was set to 10 MHz, and the gain was set to high. The use of different exposure times resulted in different frame rates.
Data and Results
The MIT researchers call attention to the fact that even though low levels of light absorption by blood and tissue, reduced scattering, and a general lack of autofluorescence can render a mouse translucent when imaged in the SWIR region, the sheer want of a versatile emitter platform has prevented widespread adoption of in vivo SWIR imaging by the biomedical research community. Their new InAs-based core/shell quantum dots (see Figure 2), however, exhibit a dramatically higher emission quantum yield (QY) than previously described SWIR probes, as well as a narrow and size-tunable emission that allows multiplexing in the SWIR region.
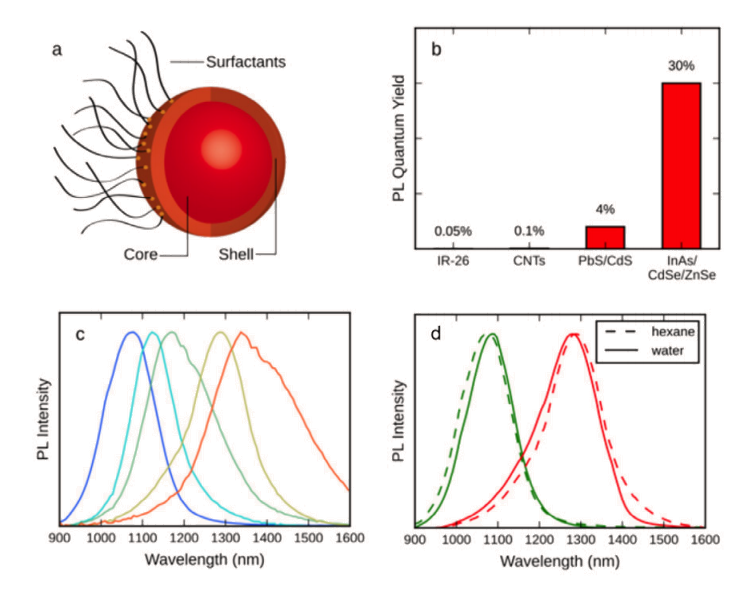
To demonstrate a few of the key capabilities of these quantum dots, SWIR imaging was utilized to measure the heartbeat and breathing rates in awake and unrestrained mice, as well as to quantify the lipoprotein turnover rates of several organs simultaneously in real-time in the mice.
The researchers also generated detailed three-dimensional quantitative flow maps of brain vasculature by intravital microscopy, visualizing the differences between healthy tissue and a tumor in the brain. These newly designed SWIR QDs enable biological optical imaging with an unprecedented combination of deep penetration, high spatial resolution, and fast acquisition speed.
For more data, as well as detailed methodologies and in-depth discussions of results, please refer to Bruns et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nature Biomedical Engineering. 2017, 1.
Although the MIT researchers did not observe toxic effects in the mice during their short-term studies, they do acknowledge that the chemical composition of their SWIR QDs might prohibit use in humans. Recently, however, they also published work showing the suitability of indocyanine green (ICG) and other substances that are clinically approved or in advanced clinical trials for SWIR bio imaging [4].
Enabling Technology
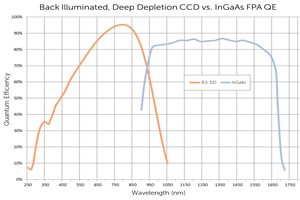
The NIRvana family of InGaAs FPA cameras from Princeton Instruments differentiates itself from other InGaAs cameras, which are typically designed for night vision applications, via a number of scientific performance features, including deep cooling, low dark noise, high linearity, low read noise, high frame rates, intelligent software, and precision control over integration times.
First and foremost, maintenance-free thermoelectric cooling chills the NIRvana 640 camera’s InGaAs detector down as low as -85°C. This deep cooling is coupled with a proprietary cold shield design and vacuum technology to facilitate the lowest possible dark noise, which helps increase sensitivity as well as preserve signal-to-noise ratio (SNR) for long exposure times when necessary
The NIRvana 640 camera has the ability to expose for as short as 2 μs, up to many minutes. Ultra-low-noise readout electronics help ensure good SNR even when the camera is operated at its maximum rate of 110 full frames per second. Furthermore, excellent camera linearity means that it is highly reliable for scientific research.
Complete control over the NIRvana camera is simple thanks to the latest version of Princeton Instruments’ 64-bit LightField® data acquisition software, available as an option. Myriad functions are provided for the easy capture and export of imaging and spectral data via the exceptionally intuitive LightField user interface. A built-in math engine can analyze the collected data in real-time. LightField also permits direct data acquisition into LabVIEW® (National Instruments) and MATLAB® (MathWorks).
Acknowledgments
Princeton Instruments would like to thank Dr. Oliver T. Bruns, Massachusetts Institute of Technology, for his invaluable contributions to this application note.
Resources
To learn more about the research being conducted by the Bawendi Lab at MIT, please visit: http://nanocluster.mit.edu/index.php
A video is also available on the Teledyne Princeton Instruments YouTube Channel, courtesy of the Bawendi Lab and MIT.
References
- Technical note: Introduction to scientific InGaAs FPA cameras. Princeton Instruments, Inc. 2012.
- Bruns et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1.
- http://chemistry.mit.edu/people/bawendi-moungi [accessed online in 2018]
- Carr et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. bioRxiv beta. April 28, 2017. https://doi.org/10.1101/100768