Introduction
For decades, x-ray and UV-vis-NIR detection methods have been used in various scientific, military, and medical applications. Although generally employed to good success, these systems nonetheless have some limitations when utilized for such types of work.
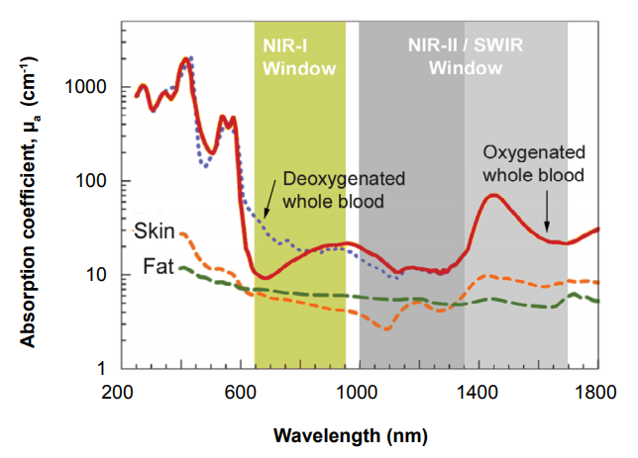
Effectively leveraging the good sample penetration afforded by x-rays, for example, can prove difficult for personnel in the field owing to the need for a compact, high-power x-ray tube as well as the lack of system portability. Alternatively, UV and visible wavelengths are quite easily detectable using silicon-based CCD technologies but are unable to penetrate samples due to reflection and scattering of light.
CCD cameras can detect NIR-I wavelengths between 750 and 1060 nm, which provide slight penetration into samples, but above this threshold the silicon itself is transparent to light. Such CCD camera systems have therefore found limited use in military (e.g., surveillance), drug discovery, and commercial (e.g., inspection) applications.
The NIR-II window / short-wavelength infrared (SWIR) range, which extends to 1700 nm, achieves much deeper penetration. Spurred on by the development of SWIR-sensitive InGaAs and InSb detectors, it has opened up a new world of scientific, military, and medical applications.

Unfortunately, early NIR-II / SWIR detection systems still had key limitations for scientific research. Insufficiencies associated with camera system linearity, noise performance, and synchronization with external equipment, as well as the lack of sufficient flexibility to control exposure times all presented critical obstacles.
This application note will discuss newly developed, deeply cooled, scientific InGaAs cameras (see Figure 1) from Princeton Instruments that facilitate leading-edge methods of small-animal imaging for preclinical research in addition to other advanced research applications.
SWIR for Small-Animal Imaging
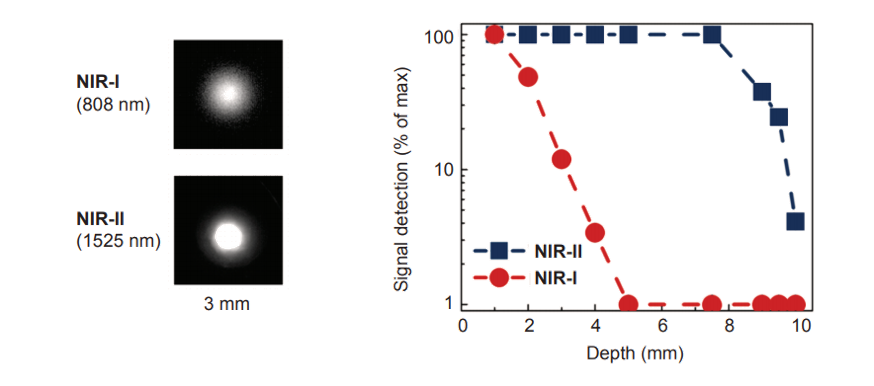
Figure 2 shows the difference between NIR-I and NIR-II / SWIR in terms of resolution and penetration depth. Notice that NIR-I wavelengths scatter, resulting in ‘fuzzier’ images. By comparison, the longer wavelengths of NIR-II / SWIR do not scatter, yielding ‘crisper’ images.
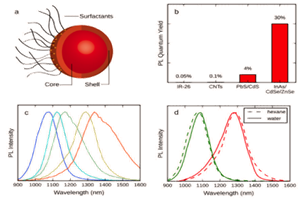
In recent years, research groups around the world have been developing different agents [1-7] to be utilized with the NIR-II / SWIR window in small-animal imaging for preclinical research, with the ultimate goal of earlier disease detection in human patients. Most of these approaches are based on one of three primary technologies: single-walled carbon nanotubes (SWNTs), rare earth–doped phosphors, or quantum dots.
SWNTs, the most mature of the three approaches, demonstrate good emissivity in the NIR-II/SWIR range and can be PEGylated to reduce toxicity [1,2]. Low-toxicity, rare-earth–doped phosphors have also shown great promise, providing emissions that are tunable to different wavelengths, dependant on the size of the nanoparticle’s undoped shell [3]. Similarly, quantum dots have demonstrated low toxicity and excellent emissivity that is tunable via particle size. (This application note focuses on the SWNT approach – for other approaches see NIR-II Probes For In Vivo Imaging.)
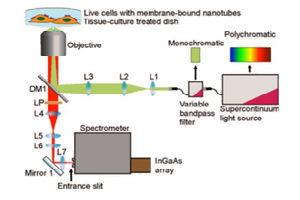
Each approach utilizes a similar experimental setup, whose main elements are NIR-I illumination, a specimen (e.g., a mouse), and a camera system to detect emitted light in the wavelength range from ~1100 to ~1650 nm. See Figure 3.
Experimental Results
Prof. Hongjie Dai at Stanford University has performed NIR-I as well as NIR-II / SWIR fluorescence imaging of blood vessels in mice [1,2]. Biocompatible SWNT-IRDye-800 conjugates were utilized as dual-color imaging agents, where IRDye-800 was a commercial NIR-I fluorophore and high-pressure carbon monoxide conversion SWNTs were stably suspended by biocompatible surfactants [1]. See Figure 4 for experimental schematics and spectra.
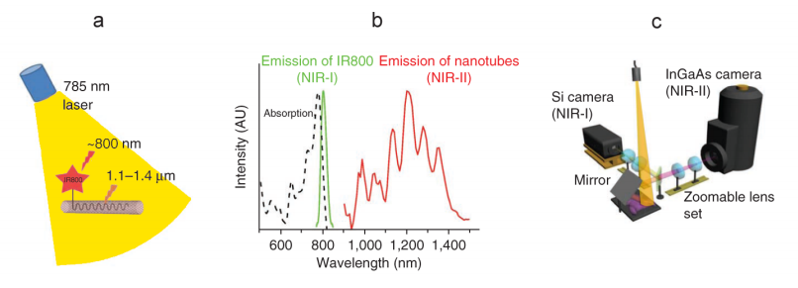
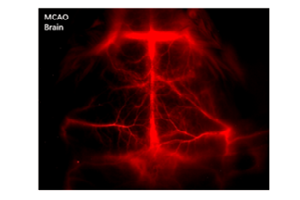
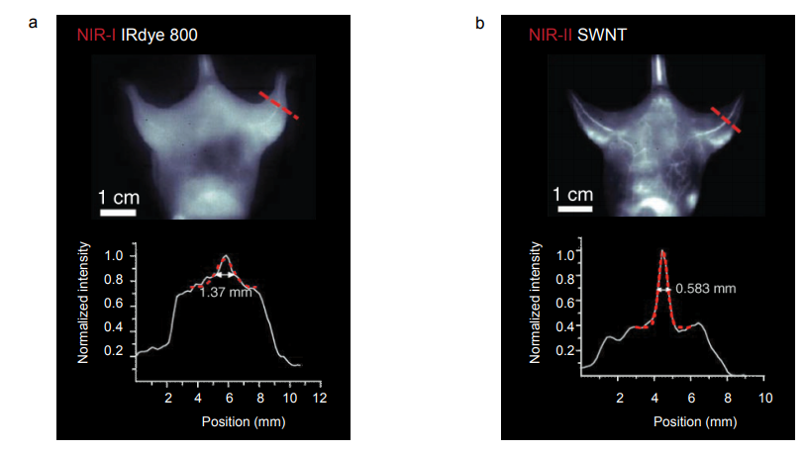
Figure 5 presents NIR-I and NIR-II / SWIR images acquired via the experimental setup shown in Figure 4c. The NIR-I image shown in Figure 5a was acquired using a scientific, silicon-based CCD camera. The NIR-II / SWIR image shown in Figure 5b was acquired using a scientific InGaAs camera. All images acquired in the NIR-I region employing IRDye-800 fluorescence showed indistinct vascular anatomy within the mouse; however, the detection of SWNT fluorescence in the NIR-II / SWIR window provided substantially improved spatial resolution of vessels in the same mouse at all magnifications [1].
New Technology
The NIRvana® family of InGaAs cameras from Princeton Instruments differentiates itself from other InGaAs cameras, which are typically designed for night vision applications, via a number of scientific performance features, including deep cooling, low dark noise, high linearity, low read noise, high frame rates, intelligent software, and precision control over integration times.
First and foremost, maintenance-free thermoelectric cooling (not liquid nitrogen) chills the NIRvana camera’s InGaAs detector down as low as -85°C. This deep cooling is coupled with a proprietary cold shield design and vacuum technology to facilitate the lowest possible dark noise, which helps increase sensitivity as well as preserve signal-to-noise ratio (SNR) for long exposure times. The NIRvana camera has the ability to expose for as short as 2 µs, up to many minutes. Ultralow-noise readout electronics help ensure good SNR even when the camera is operated at its maximum rate of 110 full frames per second. Furthermore, excellent camera linearity means
that the NIRvana is highly reliable for scientific research.
Princeton Instruments’ 64-bit LightField® software, available as an option, provides a powerful yet easy-to-use interface that puts real-time online processing capabilities at the researcher’s fingertips. The NIRvana camera can also be integrated into larger experiments using an available National Instruments LabVIEW® toolkit. Full triggering support is provided for
synchronization with external equipment.
Summary
The extension of in vivo optical imaging for disease screening and image-guided surgical interventions requires brightly emitting, tissue-specific materials that optically transmit through living tissue and can be imaged with portable systems that display data in real-time [3]. Work
performed by a number of research groups around the world has begun to demonstrate that fluorescence imaging in the NIR-II / SWIR range can provide appreciably greater in vivo sensitivity compared to fluorescence imaging in the NIR-I region [1–7].
The utilization of materials such as SWNTs, rare-earth–doped phosphors, and quantum dots in concert with deeply cooled, scientific InGaAs cameras like the new Princeton Instruments NIRvana holds great promise for the future of in vivo optical imaging applications in the NIR-II / SWIR range.
Resources
To learn more about the NIR-II / SWIR imaging techniques with SWNTs being developed by Hongjie Dai, please visit: http://dailab.stanford.edu/
References
- Hong G., Lee J.C., Robinson J.T., Raaz U., Xie L., Huang N.F., Cooke J.P., and Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat. Med. 18, 1841–1846 (2012).
- Hong G., Lee J.C., Jha A., Diao S., Nakayama K.H., Hou L., Doyle T.C., Robinson J.T., Antaris A.L., Dai H., Cooke J.P., and Huang N.F. Near-infrared II fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement. Circ. Cardiovasc. Imaging (2014).
- Naczynski D.J., Tan M.-C., Zevon M., Wall, B., Kohl J., Kulesa A., Chen S., Roth C.M., Riman R.E., and Moghe P.V. Rare-earth–doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 4, (2013).
- Welsher K., Liu Z., Sherlock S.P., Robinson J.T., Chen Z., Daranciang D., and Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 4, 773–780 (2009).
- Welsher K., Sherlock S.P., and Dai H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. PNAS 108, 8943–8948 (2011).
- Hong G., Robinson J.T., Zhang Y., Diao S., Antaris A.L., Wang Q., and Dai H. In vivo fluorescence imaging with Ag2 S quantum dots in the second near-infrared region. Angew. Chem. 124, 9956–9959 (2012).
- Tao Z., Hong G., Shinji C., Chen C., Diao S., Antaris A.L., Zhang B., Zou Y., and Dai H. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 52, 13002–13006 (2013).